Summary of Covid-19 Vaccines
Vaccines typically take years of research and testing before reaching the market. However, this has not been a typical year in any sense. Scientists and researchers are racing to produce a safe and effective coronavirus vaccine against SARS-CoV-2, the virus causing the COVID-19 pandemic. Over the past 6 months we have published reports on active Covid-19 clinical trials from around the world. This month we are publishing a summary of promising Covid-19 Vaccines.
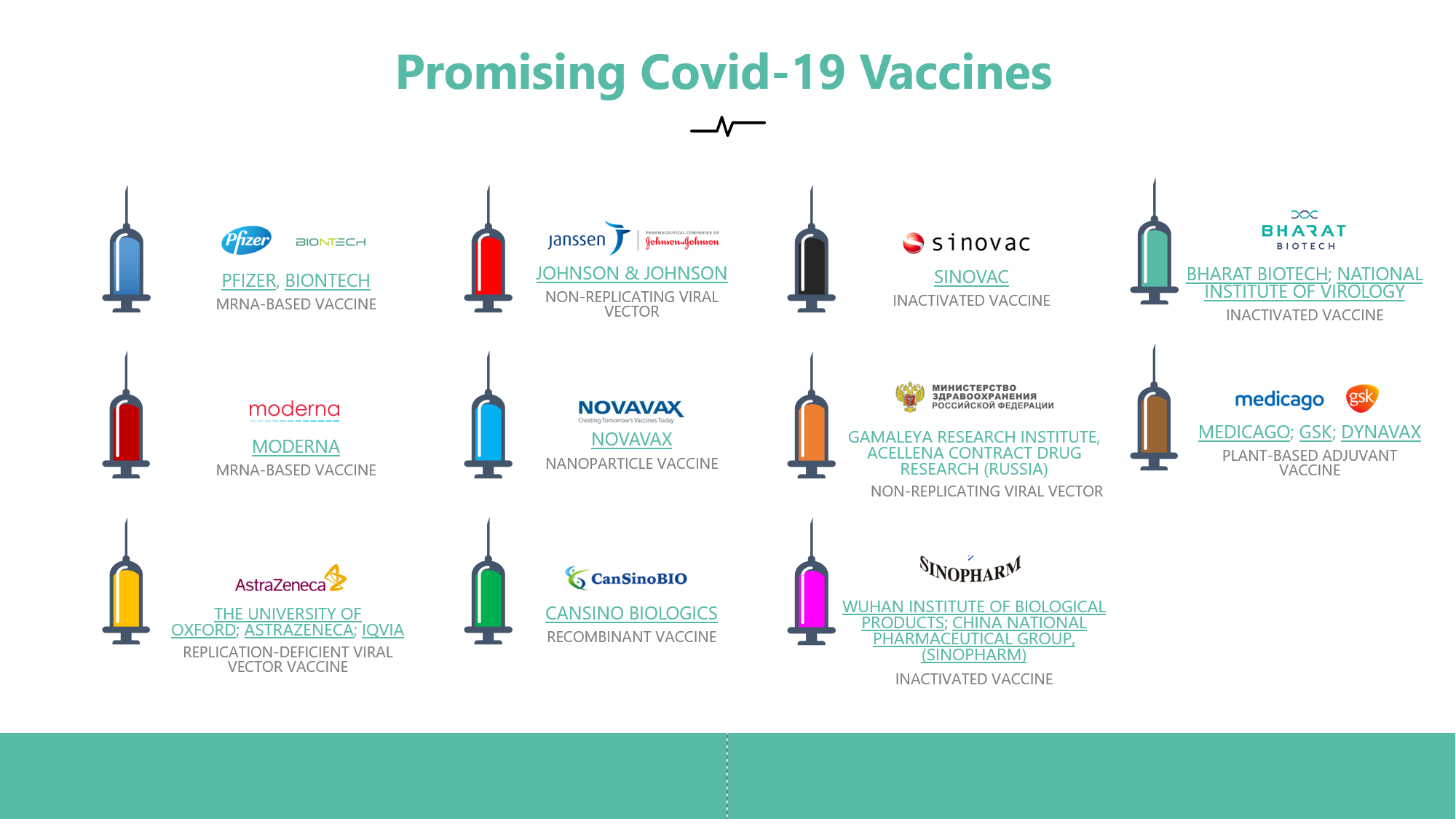
In this report, we have summarized the status of 11 promising vaccine candidates that have reached phase 3 clinical trials. We have indicated each candidate’s Trial Design, their outcomes and overall status.
This report is co-published with the drug regulatory consultancy SPharm and provided to you in a format that is hopefully useful to digest. All clinical trial data, summaries, content and references have been referenced directly from clinicaltrials.gov provided by the National Institutes of Health, by the Regulatory Affairs Professionals Society (RAPS), the New York Times Coronavirus Vaccine Tracker, and by CNN Health.
Download the Covid-19 Vaccines report.
Includes promising Vaccines from:
- Pfizer
- Moderna
- AstraZeneca
- Johnson & Johnson
- Novavax
- CansinoBio
- Sinovac
- Sinopharm
- Medicago
- Bharat Bio
- Gamaleya
TAKE THE NEXT STEP
Have any questions about your clinical trial design, protocol, or data? Ask our experts for their input or guidance. We are happy to help.
Need ballpark pricing, a comprehensive proposal, an RFP/RFI filled, review of your design, data or your development plan?
Download whitepapers, eGuides, infographics, presentations, articles and other relevant content from our resource center.