Ultimate Guide to Contract Research Organization Market Size

Ultimate Guide to Contract Research Organization Market Size
Due to Contract Research Organizations’ (CROs) significant impact on drug development and healthcare advancements, understanding the size and future growth of the CRO Market is vital. It is particularly essential now, as the demand for cost-effective and specialized research services rises.
To help present a robust view of the CRO market, we have collated data from multiple sources. Each of these sources has utilized its methodologies and datasets to represent current and future market conditions. By collecting the information from these sources, we aim to present readers with an aggregated overview of the CRO market’s size and growth rate and to give an understanding of the market’s evolution, key drivers, and potential challenges for the period of 2023 to 2028.
Table of Contents
Size and Growth of the CRO Market
We have collated data from 9 different research reports on the CRO market for the years 2023 to 2028. The following data represents the aggregate revenues from the list of CROs that each research report analyzed. The reports show that the global CRO market is estimated anywhere from USD $48.19 billion to USD $82.55 billion in 2023 and is expected to reach anywhere from USD $70.15 billion to USD $148.76 billion in 2028. The estimated growth rate for this market from 2023 – 2028 varies among reports, with predictions ranging from 6.2% to 12.5%.
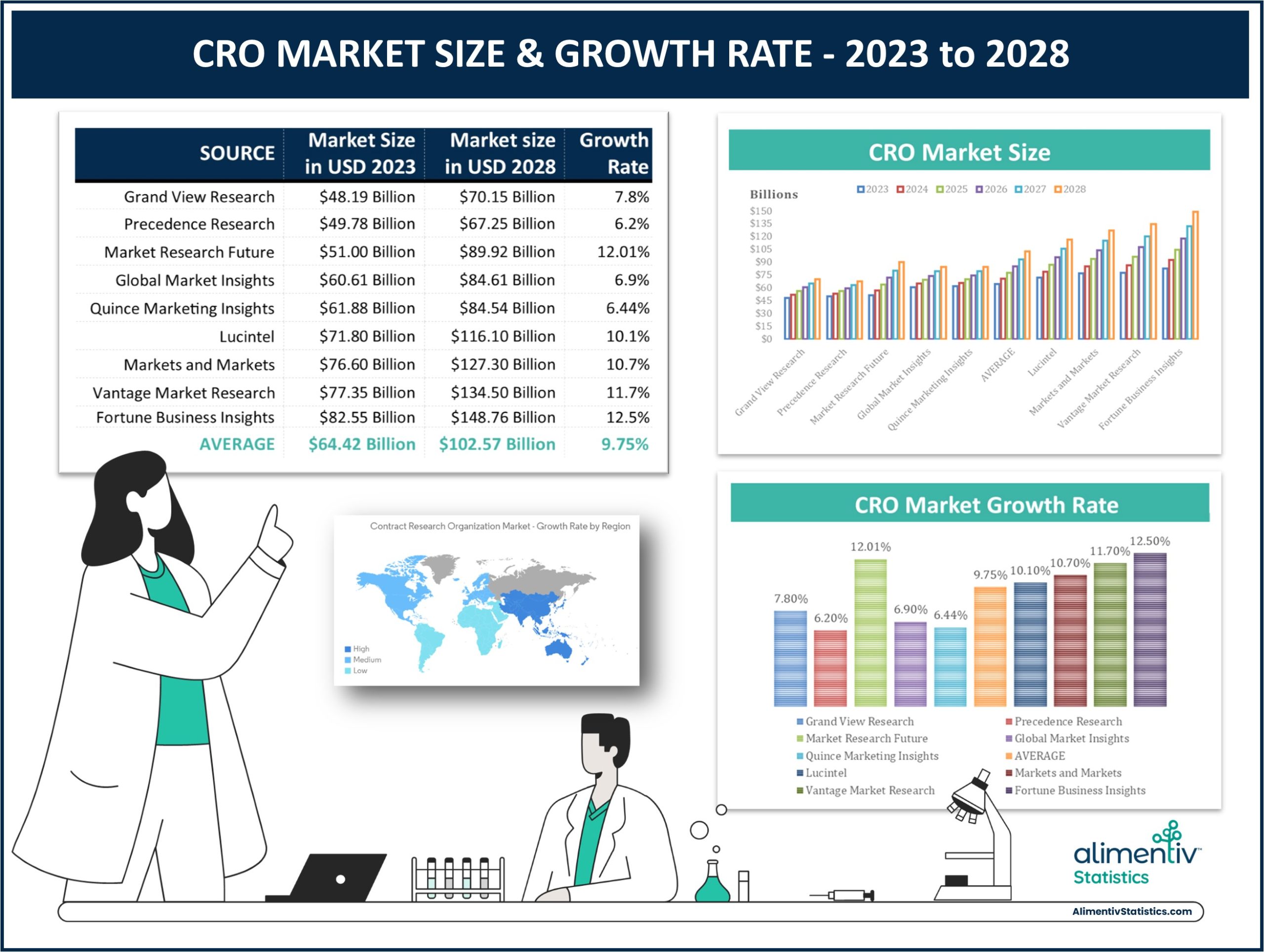
By averaging the data from all the research reports, the global contract research organization (CRO) market is estimated to average USD $64.42 Billion for 2023 and is expected to reach over USD $102.57 Billion in 2028, with an average growth rate of 9.75% from 2023 to 2028.
Across all research reports, North America was found to control a plurality of the overall CRO market with estimates anywhere from 43% to 52%. However, all sources also indicated that other regions, such as Europe and Asia-Pacific, are witnessing rapid growth, driven by an increase in clinical research activities, favourable government initiatives and the availability of skilled personnel.
Main Drivers of CRO Market Growth
We have gathered and published the main drivers of the CRO market growth over the next few years as presented in the research reports we collected. Each research report utilizes its methodologies to determine what they consider to be the main drivers of growth. The content shown below is taken directly from each research report. The explanations and paragraphs are from each report and not from us. In certain cases, we revised the sentence structure or word choice to enhance the readability of the information. Each source report is cited along with links to their report.
Source: Grand View Research
“Key factors that are driving the healthcare CRO market growth include the increasing investment in R&D programs, increasing preference for outsourcing activities due to time and cost constraints, and patent expiration of blockbuster drugs.”
Source: Precedence Research
“The rising prevalence of various chronic conditions such as cancer, diabetes, cardiovascular diseases, respiratory diseases, and musculoskeletal disorders is encouraging the pharmaceutical industry to invest heavily in the research and development of innovative and effective drugs and diagnostic devices. Two of the prominent factors driving the growth of the global healthcare CRO market are current industrial preferences for outsourcing activities to CROs to save time and costs and the expiration of patents in the healthcare sector. “
Source: Market Research Future
“The CRO market is showing exponential growth during the forecast period due to several important factors, such as the availability of funds for research; government support for research and development; well-developed healthcare sector and healthcare expenditures, and rising patient population across the world.
The key drivers of growth in the CRO industry include the growing need for cost-effective drug development processes, the rising prevalence of chronic diseases, and the surge in outsourcing to expedite research and development activities. Additionally, the increasing traction of virtual trials, remote monitoring, and telemedicine further fuels the demand for CRO services.”
Source: Global Market Insights
“Rising adoption of advanced technologies for efficient R&D outcomes and the increasing CRO outsourcing trends in the clinical trials industry has largely influenced global market growth.”
Source: Quince Marketing Insights
“The cost of treatment associated with chronic diseases is rapidly increasing on a global scale. According to the United Nations, the percentage of chronic disease-related mortality will rise to 70% and represent 56% of all deaths worldwide by 2030. Heart diseases, strokes, cancer, diabetes, obesity, arthritis, and Alzheimer’s have contributed significantly to health and economic costs. The market is anticipating substantial growth opportunities surrounding the prevalence of chronic diseases during the forecast period.”
Source: Lucintel
“The major drivers of the CRO market include the increasing need for research and development activity for drugs, the rising number of clinical trials, and the growth of private and public funding to support research activities of pharmaceutical industries.”
Source: Markets and Markets
“Factors involved in the growth of the CRO market include continuously growing pharmaceutical, biopharmaceutical, and medical device R&D pipelines and technological advancements in the clinical trials process.
Over the last decade, the drug discovery and development field has grown consistently. There has been continuous growth in the number of clinical studies conducted, resulting in novel drug molecules entering various phases of the clinical drug development cycle. According to Pharma R&D Annual Review 2022, the number of drugs in the R&D pipeline grew from 17,737 in 2020 to 20,109 in 2022. As per the ClinicalTrials.gov website, the number of registered studies went up from 32,517 in 2019 to 36,770 in 2022, at a Compound Annual Growth Rate (CAGR) of 4.2% between 2019 and 2022. This growth in the R&D pipeline of novel drugs is boosting the outsourcing of the drug development process intending to manage capacities and access scientific and process innovations to ultimately develop cost-effective and efficient drug molecules.”
Source: Vantage Market Research
“In recent years, the CRO business has experienced substantial growth, driven by the desire for speedier and more efficient drug development. Demand for clinical research services is anticipated to expand over the forecast period due to:
- the rising number of new drug launches and clinical trials globally
- rising expenditure in drug development
- an increase in outsourcing CRO services
- prevalence of chronic diseases, which is on the rise globally
The high cost of drug development is a major challenge for pharmaceutical and biotechnology companies, which are under pressure to generate returns on their investments quickly. To reduce costs these companies are increasingly outsourcing clinical research activities to CROs. Access to skilled employees, shortened time to market, and cost savings are some of the advantages of outsourcing clinical research to CROs.
Additionally, the prevalence of chronic diseases is on the rise globally. According to the World Health Organization (WHO), chronic diseases are responsible for more deaths than any other cause, and their incidence is increasing, leading to further drug development in these areas.”
Source: Fortune Business Insights
“The main drivers of growth in the CRO market include:
-The rise in the prevalence of chronic disease.
According to the UN’s Food and Agriculture Organization, chronic diseases accounted for approximately 60% of the world’s 56.5 million documented deaths and 46% of the global disease burden. Chronic disease is a problem that affects people worldwide, not only in developed countries. The increasing prevalence of chronic diseases and rising awareness against these diseases have increased the pressure on the market players to develop efficient medications and are expected to boost the market significantly.
-An increase in the number of clinical trials
Recently, the number of clinical trials that have been filed has risen significantly. This increase in the number of clinical trials is anticipated to boost the development of new drugs and is expected to drive the CRO market’s growth in the forecast period.”
Opportunities for CRO Market Growth
We have gathered and published the opportunities for CRO market growth over the next few years as presented in the research reports we collected. Each research report utilizes its methodologies to determine what they consider as opportunities for growth. The content shown below is taken directly from each research report. The explanations and paragraphs are from each report and not from us. In certain cases, we revised the sentence structure or word choice to enhance the readability of the information. Each source report is cited along with links to their report.
Source: Grand View Research
“-Increasing pressure on drug developers pertaining to clinical data management, regulatory environments, and stringent safety standards are expected to drive demand for contract research organizations.
-The Covid pandemic created a need for virtual trials, thereby leveraging the usage of digital technology & software solutions. The growth in the adoption of machine learning-based platforms, artificial intelligence, and other digital and mobile technologies are opportunities for future expansion.
-A rise in collaborations amongst CROs and biopharmaceutical companies or other sponsors is fuelling the co-opetition movement.”
Source: Market Research Future
“-The opportunities for growth in the CRO sector lie in expanding therapeutic expertise to cater to emerging treatment areas and rare diseases.
-The adoption of artificial intelligence and big data analytics in clinical trials presents an opportunity for CROs to enhance efficiency and deliver more precise results.
-The trend of biopharmaceutical companies outsourcing early-phase development and regulatory consulting creates new avenues for CROs to diversify their service offerings
-Rising investment by biopharmaceutical and medical device companies in the creation of new drugs and technologies. The global market is being fueled by rising investments made in clinical and non-clinical research activities, as well as by outsourcing to numerous contract research organizations services that help give cost-effective development options.”
Source: Global Market Insights
“-The significant shift from maintaining manual/paper-based records to digital data capture technologies is largely transforming and propelling the market growth.
-The Covid pandemic triggered the adoption of digital resources across clinical trials. These digital technologies transform the approach towards clinical development by incorporating valuable insights from multiple sources and increasing the volume of data collected in trials, enhancing clinical trial productivity.
-Growing number of clinical trials in emerging countries.
-Increasing R&D expenditure worldwide will spur the CRO industry growth.
The increasing burden of chronic diseases worldwide is expected to propel the contract research organization market growth over the forecast period. Sedentary lifestyle, unhealthy eating habits, consumption of tobacco and smoking are some of the prominent factors contributing to the increasing burden of chronic diseases.”
Source: Quince Marketing Insights
“-Tech-Enabled Patient Recruitment & Management. Patient recruiting and management provides one of the largest issues for clinical research organizations, locations, and sponsors. In the past years, patient recruitment mainly relied on manual procedures including fliers, newspaper ads, and physician referrals. To reach a larger pool of possible study participants, CROs increasingly use digital channels with the development of new technology. Electronic data capture (EDC), which collects clinical trial data electronically rather than through paper forms, is another major technological advance in this field. EDC solutions can improve data accuracy and completeness while streamlining the data collection process. With EDC, CROs can manage data from multiple sites, collect patient data remotely, and guarantee better data integrity throughout the trial. Additionally, CROs can use other technologies, such as social media and mobile apps, to increase patient engagement and recruitment.”
Source: Markets and Markets
“-The growing demand for new clinical trial designs for cell & gene therapies is expected to offer growth opportunities to companies operating in this market. Cell & gene therapies are complex and very specific and are perceived to address unmet medical needs. This has led to increased investment in developing and commercializing new cell & gene therapies. The growing number of cell therapy candidates, coupled with their rapid progression through the various phases of clinical development, has increased the demand for facilities that offer R&D services for these therapies. These factors are expected to drive the CRO services market.”
Source: Vantage Market Research
“-The use of technology is becoming increasingly important in clinical research, with eClinical solutions and electronic data capture (EDC) systems seeing rapid adoption. This is helping to speed up clinical trials and improve data quality.
-There has been consolidation in the CRO industry in recent years, with large players acquiring smaller CROs to expand their geographical reach and service offerings. This trend is expected to continue in the coming years.
-There is strong growth potential in emerging markets such as Asia-Pacific, Latin America, and Eastern Europe, as these regions have a large patient population and are seeing an increasing number of clinical trials.
-Outsourcing of clinical trials is increasing. As drug development costs continue to rise, more and more pharmaceutical companies are outsourcing their clinical trials to CROs. This trend is expected to continue as CROs become increasingly specialized and efficient at running clinical trials.
-The use of data analytics in clinical trials is on the rise. With the ever-increasing volume of data being generated in clinical trials, CROs are turning to data analytics to help them make sense of it all. Data analytics can be used to improve trial design, identify risk factors for patient drop-out and predict which patients are most likely to respond to a particular treatment.”
Source: Fortune Business Insights
“The CRO market is expected to continue to grow in the future due to several factors, including the increasing number of new drug approvals, the growing demand for personalized medicine and the increasing focus on drug safety and efficacy.
-Sponsors generally outsource therapeutic and other product development functions to independent service providers, which leads them to use a more flexible cost structure and avoid maintaining redundant development capabilities worldwide. Increasing investments in outsourcing to many contract research organizations will continue to fuel the global market growth.
-The trend shows that pharma companies are enhancing their R&D efficiencies and collaborating on added R&D The pharma R&D sector is changing as companies are focused on making trial endpoints more patient-centric and responsive. These factors will augment the market growth.”
Challenges in the CRO Market
Source: Quince Marketing Insights
“One of the main challenges in the growth of the Contract research organization market is the high-quality standards CROs must fulfil to be qualified to carry out studies. There are international standard operating procedures (SOPs) to adhere to, system audits to be conducted and all staff involved in the trials should be trained to follow Good Clinical Practice (GCP), local regulations and other guidelines. Although the sponsor is always ultimately responsible for the accuracy and reliability of the trial data, CROs can overcome this challenge by adhering to quality assurance and quality control requirements.”
Source: Vantage Market Research
“There are several key constraints that have been preventing the growth of the Contract Research Organization Market.
-Many pharmaceutical and biotechnology companies are hesitant to outsource their research and development (R&D) activities to CROs, as they are concerned about losing control over their intellectual property.
-Regulatory pressures are increasing worldwide. As clinical trials become more global, CROs must comply with a growing number of regulations, which are increasingly time-consuming and expensive.
-Patient recruitment and retention are becoming increasingly challenging – and important. With longer and more complex clinical trials becoming the norm, recruiting and retaining patients has become a major challenge. While traditional methods such as direct mail and newspaper ads are still used, CROs are increasingly turning to social media and online advertising to reach patients.”
Source: Markets and Markets
“One of the major challenges for CROs and life science companies is Patient recruitment and retention. The main challenges related to patient recruitment include protocol complexities and competition in clinical trials, which makes it increasingly hard to find qualified patients. Moreover, a lack of understanding of the patient’s perspective is another factor that makes it difficult for recruiters to retain patients throughout the trial. However, decentralized clinical trial services are likely to reduce the effects of this challenge over the coming years. Many rely heavily on offline recruitment through marketing in newspapers, public transport, or street posters, others rely on doctors and study center recruitment. Some of these challenges can be overcome through the use of social media and various other online marketing channels.”
Source: Global Market Insights
“Intellectual property issues are one of the major concerns faced in outsourcing clinical trials that may potentially slow down the contract research organization industry growth. Most medical devices and drug candidates are patented products and outsourcing to contract research organizations may increase the chances of data leakage.”
Source: Fortune Business Insights
“The CRO market faces several challenges, including the high cost of clinical trials, increased regulatory complexity and the lack of skilled professionals. All of which may strain the growth of the market. CROs face issues attracting and maintaining highly competent experts as they compete for qualified and experienced scientists with biotechnology, pharmaceutical, medical devices businesses, and academic and research institutions. This scarcity of qualified specialists is a barrier to adopting novel procedures and technologies, restricting market growth.”
Source: Market Research Future
“Challenges facing the industry include stringent regulatory requirements, high cost of labour, economic volatility, data privacy concerns, and the increasing complexity of clinical trial designs. Furthermore, the CRO market’s competitive landscape is intense, with both global and regional players vying for contracts, necessitating a focus on differentiation and innovation to maintain a competitive edge. The market’s high cost of labour is a problem that could hinder expansion within the anticipated time range. The high cost has caused demand to wane in several markets.”
Final Thoughts on CRO Market Growth
Overall, all of the cited reports provided a positive outlook for the global Contract Research Organization industry. A summary of the most frequent findings include:
Size and Growth: By averaging the data from all the research reports, the global contract research organization market is estimated to average USD $64.42 Billion for 2023 and is expected to reach over USD $102.57 Billion in 2028, with an average growth rate of 9.75% from 2023 to 2028. The North American CRO market is the largest in the world, accounting for anywhere from 43% to 52% of the global market. However, the Asia-Pacific region’s market is expected to grow at the fastest pace over the next few years.
Drivers of Growth: The CRO market is expected to grow at a rapid pace due to several factors, including the increasing complexity of drug development, the rising cost of clinical trials, the growing demand for clinical trial data, and the increasing use of outsourcing of R&D by pharmaceutical companies.
Despite the cited challenges, the industry’s adaptability, coupled with the rising demand for specialized services and continuous technological advancements, presents opportunities for CRO companies to expand and contribute to the advancement of pharmaceutical research and development worldwide.
Featured Posts
CONTACT
ALIMENTIV STATISTICS
Providing 40 years of service, Alimentiv Statistics is a niche Contract Research Organization focused on Biostatistics, Statistical Analysis, Clinical Trial Design and Data Management. We invite you to see how we exceed our clients’ expectations.